Good Documentation Practices Fda
How To Implement Good Documentation Practices
good documentation practices fda is free HD wallpaper was upload by Admin. Download this image for free in HD resolution the choice "download button" below. If you do not find the exact resolution you are looking for, then go for a native or higher resolution.
Don't forget to bookmark good documentation practices fda using Ctrl + D (PC) or Command + D (macos). If you are using mobile phone, you could also use menu drawer from browser. Whether it's Windows, Mac, iOs or Android, you will be able to download the images using download button.
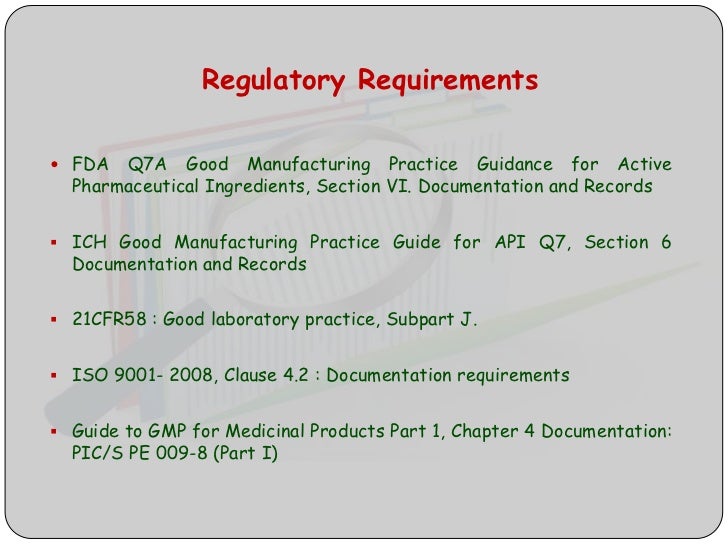
 In Fda Regulated Industries Good Documentation Practices
In Fda Regulated Industries Good Documentation Practices
 In Fda Regulated Industries Good Documentation Practices
In Fda Regulated Industries Good Documentation Practices
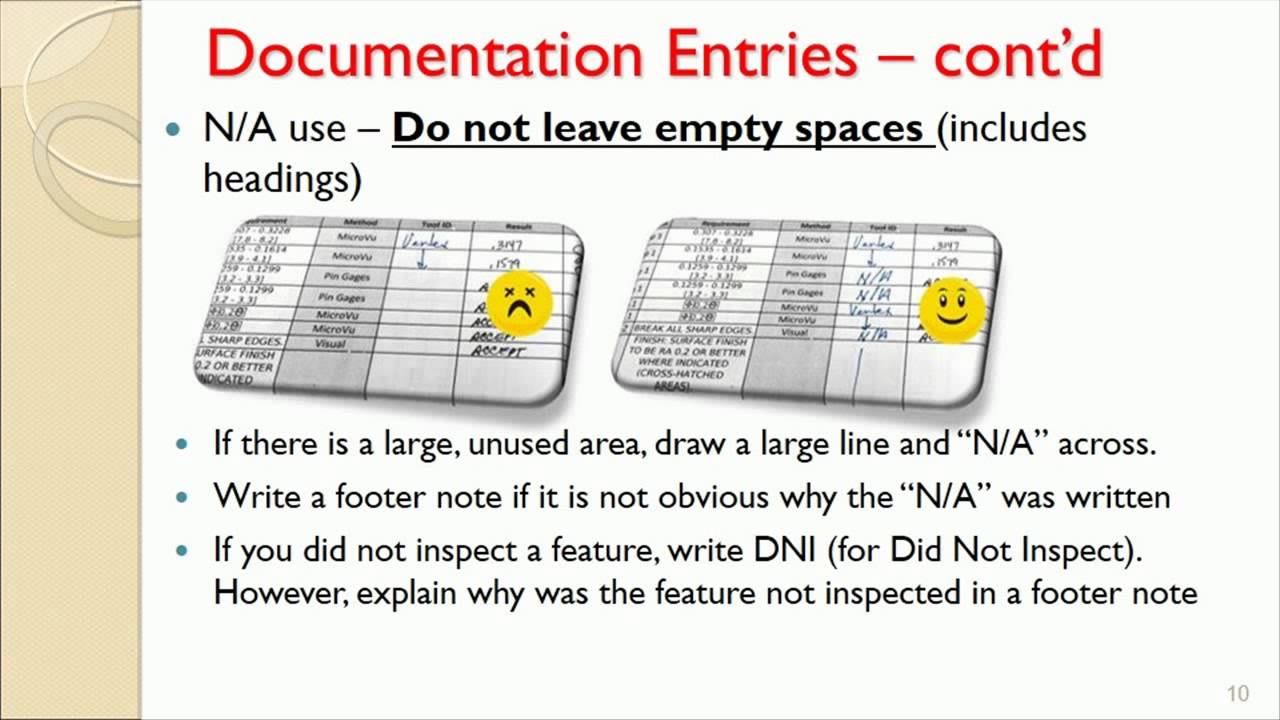 Good Documentation Practices 10 7 2014
Good Documentation Practices 10 7 2014
 Good Documentation Practices Do S And Don Ts Gcp
Good Documentation Practices Do S And Don Ts Gcp
How To Implement Good Documentation Practices
Data Integrity And Good Documentation Practices Not Just
 Pdf Good Documentation Practices Gdps In Pharmaceutical
Pdf Good Documentation Practices Gdps In Pharmaceutical
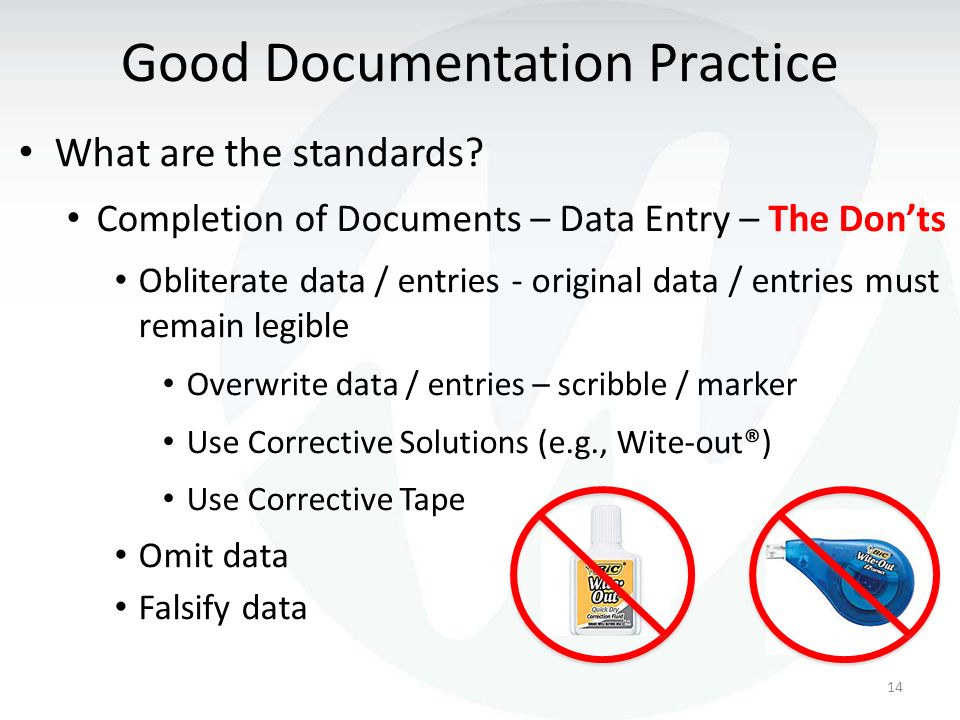 In Fda Regulated Industries Good Documentation Practices
In Fda Regulated Industries Good Documentation Practices
How To Implement Good Documentation Practices
 Good Documentation Practices Gdps For Fda Regulated
Good Documentation Practices Gdps For Fda Regulated
 Strategies For Ensuring Good Documentation Practices Gdp Trailer
Strategies For Ensuring Good Documentation Practices Gdp Trailer
 Good Documentation Practices To Support Computer System Validation
Good Documentation Practices To Support Computer System Validation
Good Documentation Practices Gdp Training
 Good Documentation Practices Gdp 101 Webinar Medical
Good Documentation Practices Gdp 101 Webinar Medical
 Gdp Good Documentation Practices
Gdp Good Documentation Practices
 Good Documentation Practices To Support Fda Computer System
Good Documentation Practices To Support Fda Computer System
 In Fda Regulated Industries Good Documentation Practices
In Fda Regulated Industries Good Documentation Practices
 Gmp02 Good Documentation Practice Zenosis Learning For Life
Gmp02 Good Documentation Practice Zenosis Learning For Life
 Pdf Good Documentation Practices
Pdf Good Documentation Practices
 Alcoa In Pharmaceuticals A Necessary Tool For Quality
Alcoa In Pharmaceuticals A Necessary Tool For Quality
 Good Documentation Practices Gdps For Fda Regulated
Good Documentation Practices Gdps For Fda Regulated
Current Expectations And Guidance Including Data Integrity

 Best Practices Implementing Computer System Validation
Best Practices Implementing Computer System Validation
 Good Documentation Practices Gdocp And Gdp Training Gdp
Good Documentation Practices Gdocp And Gdp Training Gdp
Data Integrity And Good Documentation Practices Not Just
 Good Documentation Practices In Pharmaceuticals
Good Documentation Practices In Pharmaceuticals
How The Ich S New Inspection Protocol Program
 Good Documentation Practice Guideline Is Simple Just Write
Good Documentation Practice Guideline Is Simple Just Write
 Good Documentation Practices An Honest Discussion On
Good Documentation Practices An Honest Discussion On
 Webinar From Traininng Com About Good Documentation And
Webinar From Traininng Com About Good Documentation And
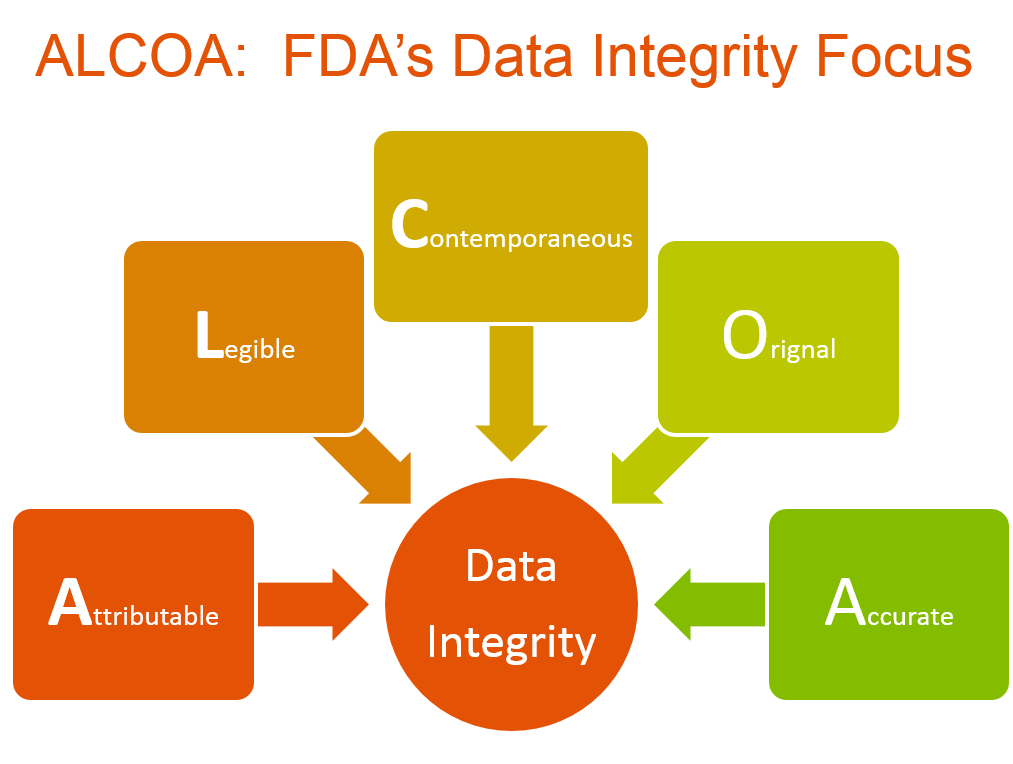 Data Integrity Foundation Of Compliance Critical Source Of
Data Integrity Foundation Of Compliance Critical Source Of
 Which Areas Do Good Documentation Practice Guidelines Affect
Which Areas Do Good Documentation Practice Guidelines Affect
 Good Documentation Practices General Rules
Good Documentation Practices General Rules
 Good Documentation Practices To Support Fda Computer System
Good Documentation Practices To Support Fda Computer System
 Data Integrity And Good Documentation Practice Eca Academy
Data Integrity And Good Documentation Practice Eca Academy
Good Documentation Practices Gdp 101 Webinar Medical
 Concept Of Gxp In Pharmaceuticals Pharmaceutical Guidelines
Concept Of Gxp In Pharmaceuticals Pharmaceutical Guidelines
Quality Medical Regulations Services Qmrs
 Qc Laboratory Documentation To Ensure Fda Compliance
Qc Laboratory Documentation To Ensure Fda Compliance
 Good Documentation Practices Kymanox
Good Documentation Practices Kymanox
 Data Integrity And Good Documentation Practices Gdp
Data Integrity And Good Documentation Practices Gdp
 Proper Documentation Practices Tips For Documenting Food
Proper Documentation Practices Tips For Documenting Food
 Calameo Good Documentation Practices For Gmp Operations
Calameo Good Documentation Practices For Gmp Operations
Review Article Coden Ijprnk Issn 2277 8713
Good Documentation Practices Gmpviolations
 Good Documentation Practices To Support Fda Computer System
Good Documentation Practices To Support Fda Computer System
Good Documentation Practice Gdp
Fda Update The Fda S New Drug Approval Process
How Labview Code Can Be Fda Validated Complying With
![]() Fda Compliance For Pharmaceuticals Regulatory Planning
Fda Compliance For Pharmaceuticals Regulatory Planning
 Why Gmp An Explanation Of Good Manufacturing Practice
Why Gmp An Explanation Of Good Manufacturing Practice
 Data Integrity Compliance With Gmp And Fda Requirements
Data Integrity Compliance With Gmp And Fda Requirements
Quality Of Medicines Deficiencies Found By Brazilian Health

 The Differences Between Gcp Glp And Gmp Audits
The Differences Between Gcp Glp And Gmp Audits
 Documentation Resume Samples Velvet Jobs
Documentation Resume Samples Velvet Jobs
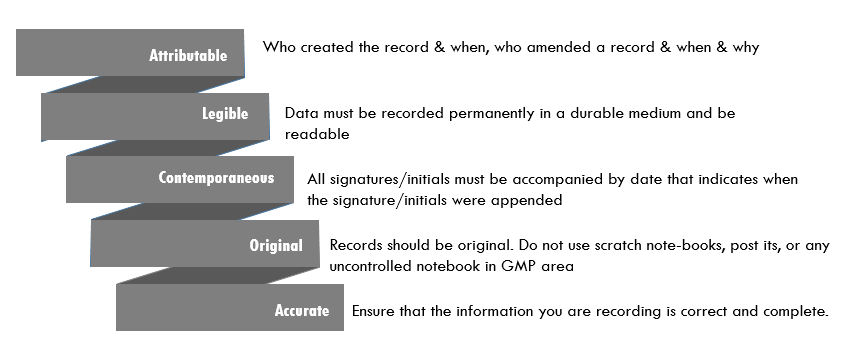 Regulatory Expectations For Data Integrity
Regulatory Expectations For Data Integrity

 Elm 104 Good Documentation Practices
Elm 104 Good Documentation Practices
 Good Documentation Practice Guideline Is Simple Just Write
Good Documentation Practice Guideline Is Simple Just Write
 Good Manufacturing Practice Course Gmp Program Cfpie
Good Manufacturing Practice Course Gmp Program Cfpie
 Class Notes Lecture 6 Validation And Regulatory Issues In
Class Notes Lecture 6 Validation And Regulatory Issues In
 Good Documentation Practices Gdp Ppt Video Online Download
Good Documentation Practices Gdp Ppt Video Online Download
 White Paper How To Implement Good Documentation Practices Pdf
White Paper How To Implement Good Documentation Practices Pdf
 8 Tips To Comply With Fda 21 Cfr Part 11
8 Tips To Comply With Fda 21 Cfr Part 11
 What Is Good Manufacturing Practice Definition And Meaning
What Is Good Manufacturing Practice Definition And Meaning
Single Registration Good Documentation Practices For Fda
 Cgmp Documentation And Record Keeping An Abridged Course
Cgmp Documentation And Record Keeping An Abridged Course
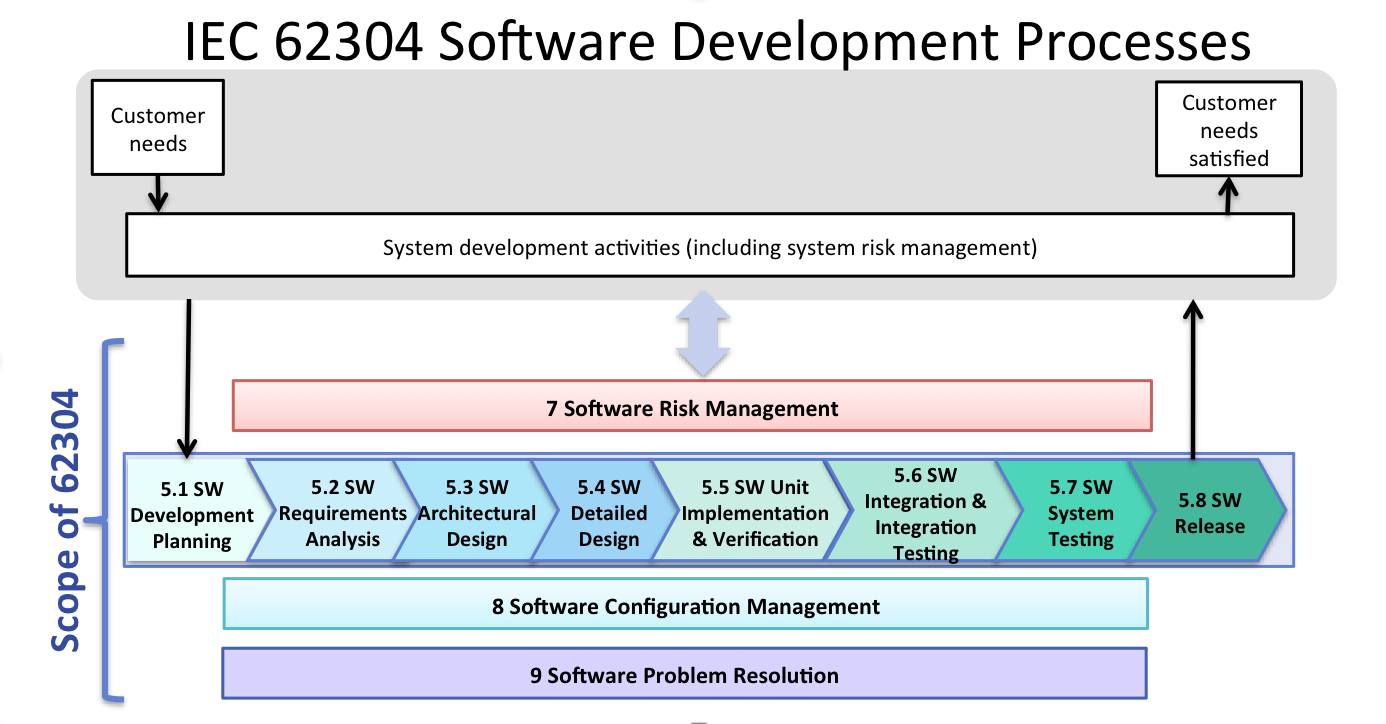 Fda Software Guidances And The Iec 62304 Software Standard
Fda Software Guidances And The Iec 62304 Software Standard
 Complianceonline On Twitter Good Manufacturing Practices
Complianceonline On Twitter Good Manufacturing Practices
 Good Manufacturing Practice Wikipedia
Good Manufacturing Practice Wikipedia
 Pin By Online Compliance Panel On Fda Compliance Webinar
Pin By Online Compliance Panel On Fda Compliance Webinar
 Good Documentation Practices Gdps For Fda Regulated
Good Documentation Practices Gdps For Fda Regulated
 Elm 502 Good Documentation Practices Corrections
Elm 502 Good Documentation Practices Corrections
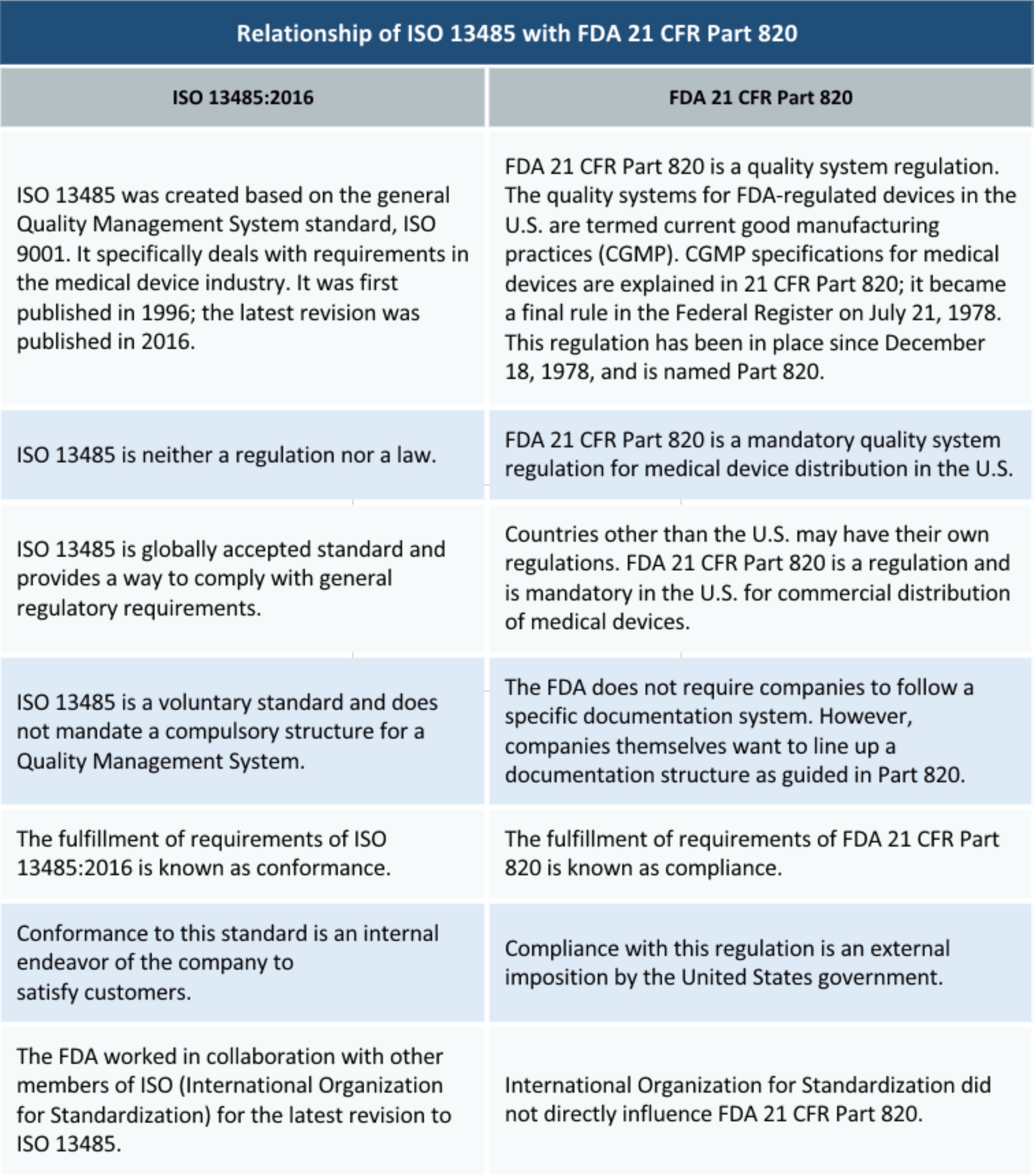 Fda 21 Cfr Part 820 Vs Iso 13485 Differences Similarities
Fda 21 Cfr Part 820 Vs Iso 13485 Differences Similarities
 Good Documentation Practices Do S And Don Ts Gcp
Good Documentation Practices Do S And Don Ts Gcp
Morf Media Inc Launches Good Documentation Practices Course
 Good Documentation Practices An Honest Discussion On
Good Documentation Practices An Honest Discussion On
.jpg) Good Documentation And Record Keeping Best Practices Fda Ema
Good Documentation And Record Keeping Best Practices Fda Ema
 Agile In Government Keeping Documentation Lean Agile Alliance
Agile In Government Keeping Documentation Lean Agile Alliance
Akorn Inc Somerset Nj 483 Issued 08 30 2018
 Pdf Good Documentation Practice In Clinical Research
Pdf Good Documentation Practice In Clinical Research
 The Necessity Of Clinical Research Documentation Training
The Necessity Of Clinical Research Documentation Training
How To Implement Good Documentation Practices
 Good Documentation Practices Gdp
Good Documentation Practices Gdp
Good Documentation Practice Gdp
 Good Documentation Practices Kymanox
Good Documentation Practices Kymanox
 Gmp02 Good Documentation Practice Zenosis Learning For Life
Gmp02 Good Documentation Practice Zenosis Learning For Life